Each year on May 15, the international community celebrates MPS Awareness Day, to raise awareness and recognize the resilience of communities affected by all types of mucopolysaccharidosis or MPS disorders. Mucopolysaccharidoses are a group of rare genetic metabolic diseases caused by the absence or malfunctioning of certain enzymes the body needs to break down molecules called glycosaminoglycans (GAGs). This can lead to progressive damage in many organs if untreated. Ultragenyx has a deep commitment to the MPS community, and currently has one approved treatment and one investigational therapy for two types of MPS disorders.
One of the rarest forms of the disorder is MPS VII, also known as Sly syndrome. It is estimated that fewer than 1 in 1,000,000 people are living with MPS VII – a population of only a few hundred people worldwide. To learn more about the unique experiences of the MPS VII community, I sat down with Terri Klein, president and CEO of the National MPS Society and Helen Evangelista, whose son Matthew is living with MPS VIII and was the first person to receive enzyme replacement therapy for the disease.

Photo (left to right): Terri Klein and Helen Evangelista
Belen: Could you both share a little about your connection to the MPS community?
TERRI: I’m the President and CEO of the National MPS Society. I’m also a parent of a young adult with Mucolipidosis Type III. Our youngest daughter was diagnosed in 1999. She’s now 31 years old and a patient scientist. There is no treatment for ML – we simply manage symptoms as they arise.
HELEN: My son Matthew was diagnosed with MPS VII, a rare genetic disorder, at the age of one year and four months. It was a devastating moment for me as there is no cure for MPS VII. Thankfully, Matthew is doing well now.
Belen: Terri, let’s start by understanding what MPS VII is and how the National MPS Society is advocating for families living with MPS VII in the US.
TERRI: MPS VII, also known as Sly syndrome, is one of the rarest lysosomal storage disorders. It is a genetic disorder that affects various body systems and can cause progressive damage to organs such as bones and joints. People living with MPS VII are missing a specific enzyme called B-glucuronidase, which leads to the accumulation of glycosaminoglycans (GAGs) in their cells. As a progressive disease, MPS VII poses significant challenges for patients and their families.
MPS VII, also known as Sly syndrome, is one of the rarest lysosomal storage disorders.
The National MPS Society has been advocating for MPS and ML for 49 years, working with policy makers to push for crucial federal legislation that supports rare disease patients, including those with MPS VII. We also focus on initiatives to identify patients early and address their unmet needs in a comprehensive manner, including newborn screening. Newborn screening programs are state-run public health programs that identify newborns with certain genetic, metabolic, hormonal or functional disorders and can lead to early detection and diagnosis. There are now two MPS disorders included on the national list known as the Recommended Uniform Screening Panel (RUSP) – MPS I and MPS II. Getting MPS VII added to the screening panel is a challenge, and we’re committed to making it happen.
Belen: When families get diagnosed with MPSVII and reach out to the National MPS Society, what type of resources do you provide them?
TERRI: The National MPS Society has a unique program called Pathways that provides support to MPS VII families. When a family reaches out to us, our team of licensed social workers meets with them directly at the time of diagnosis. Our social workers offer support beyond the hospital setting, helping families navigate their diagnosis and life as a whole. MPS VII can be overwhelming, and having someone to lean on and provide comprehensive support can help alleviate some of the burden for families.
In addition, the National MPS Society provides educational materials, information about clinical trials and research, financial assistance programs, and a supportive community through events and online platforms. We also collaborate with medical professionals and researchers to advance the understanding and treatment of MPS VII, and we work towards increasing awareness and advocacy at the national and international levels.
Ongoing research has the potential to change everything for the MPS community.
Belen: How does ongoing research in MPS VII impact the patient community?
TERRI: Ongoing research has the potential to change everything for the MPS community. Research has the potential to uncover new therapies and treatments that significantly impact the future for these families. The patient community who are participating in research today are changing the future for the next generation.
Belen: What is the role of the MPS Society internationally?
TERRI: The National MPS Society plays a role on the international stage by collaborating with the International MPS Network to understand the unmet needs of MPS patients in different countries. The Society works on developing educational materials, such as booklets and resource guides, which can be translated into different languages and made accessible to clinics, geneticists and doctors around the world. This allows for the dissemination of information and support for MPS and MPS VII patients globally.
Belen: Helen, could you tell us a little bit about your story and Matthew’s journey with MPS VII?
HELEN: Matthew was very sick when he was born, but doctors couldn’t figure out what was wrong with him as all the tests came back negative. He spent four months in ICU and had to be discharged because the insurance wouldn’t cover it anymore, which was very scary. We brought him home with a monitor, but he continued to have frequent hospital visits due to severe respiratory problems.
The doctor came to me and said, ‘Your son is diagnosed with MPS VII.’ I said, ‘What is MPS VII?’ They handed me a pamphlet and told me there was no cure.
Matthew was one year and four months old when he was finally diagnosed with MPS VII. He was doing really well at home, but all of a sudden, he fell inside his crib and then he couldn’t move his arms or legs. I was devastated. We took him to another hospital where they performed a genetic test, and the doctor came to me and said, “Your son is diagnosed with MPS VII.” I said, “What is MPS VII?” They handed me a pamphlet and told me there was no cure.
Belen: How did you feel when you heard the diagnosis of MPS VII
HELEN: It was a shock for me when the doctor told me that there is no cure for MPS VII. I didn’t fully understand the sickness at first so I was reading everything I could find about it. My family members hoped that Matthew would grow up and everything would change, but the doctor explained that MPS VII is part of “the blueprint of his body,” so he would not grow out of it.
I read that stimulation was really important, so I made sure to stimulate him every way I can, when we were reading a book, I wanted him to feel what the characters were feeling, I played music for him, I took him outside to feel the sun, and I had all the therapies done in our house, all to stimulate him in those early years of his life. I reached out to the MPS Society, and I went to MPS Society conferences to learn how to take care of Matthew. And at first, I was the only person representing MPS VII at these events because it is so rare.
He was living in the hospital most of the time and I could bring him home on the weekends at first, but then as he grew and as he became more dependent on a ventilator, we could no longer take him home, so he lives full time in the hospital now.
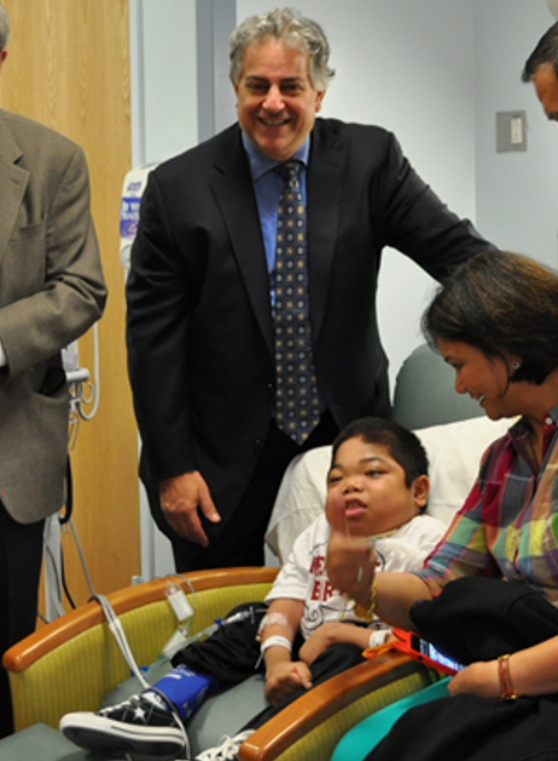
Photo: Matthew receiving the first-ever treatment infusion in 2013
Belen: How did you decide to participate in the clinical trial for MPS VII?
HELEN: When I heard about the clinical trial, I decided I wanted to do something for him, you know? Even when people would say, “Are you sure? Are you going to do it? Because once you put that medicine in his body, you don’t know what’s going to happen to him – his body might shut down and he could die.”
I told them I’d rather try than not try. I mean, what if it works?
I told them “I’d rather try than not try. I mean, what if it works?” And even if it doesn’t work, I want him to at least have a fight, you know? I believed that trying the clinical trial was worth it. I didn’t want to give up without trying everything possible to help my son.
We got FDA permission to have Matthew join the clinical study because he was in critical condition, and he became the first-ever patient to receive the enzyme-replacement therapy for MPS VII. He has been on the medication ever since.
Belen: How has Matthew’s life been since the clinical trial?
HELEN: Matthew just turned 21, and everybody is so happy he has been able to celebrate his birthday and enjoy life despite the challenges of MPS VII.
Video: Matthew’s 21st birthday celebration —
Belen: What is your hope for the future for individuals and families living with MPS VII?
TERRI: Ultragenyx is running a disease monitoring program for MPS VII, which goes beyond a natural history study and includes monitoring treated and untreated patients. The program gathers data from areas such as patient- and caregiver-reported outcomes and antibodies to treatment. This program will provide clearer information about the disease and help educate families, scientists, and researchers. The data gathered from the disease monitoring program, along with stories like Matthew’s, will be important for the families of the future.
HELEN: You know, I’m very happy that we’re giving more hope to other families. And I’m so happy every time I get a call from another MPS VII family asking me about our experience – I share what I know and I’m open to anyone who has been diagnosed with MPS VII. I’m here and willing to give all the information that they need.
Belen Gonzalez Sutil is director of patient advocacy at Ultragenyx





